Vero Cell Covid Vaccine Efficacy Rate | Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
Sinopharm vero cell vaccine efficacy. The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported.

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation.

Vero cell covid vaccine efficacy rate. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Vaccine Efficacy 10000 CI 95 2033-10000 Efficacy against symptomatic COVID-19 cases in Indonesia Efficacy after 14 days with two doses of vaccination Data as of January 11 2021. So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
To estimate efficacy at preventing disease we use the result from the corresponding clinical trials as documented in Table 1. On December 29 2020 Sinopharm reported 79. Vero cell vaccine efficacy china.
10 11 Peer-reviewed results published in JAMA of Phase III trials in United Arab Emirates and Bahrain showed BBIBP-CorV 781 effective against symptomatic cases and 100 against severe cases 21 cases in vaccinated group vs. 95 e02260-20 2021. The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning.
Doubts Over China Vaccines Effectiveness Mar. The two jab vaccine is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Cells used for vaccine manufacture are the working Vero cell bank which is of the 142nd generation.
In addition we modify the effectiveness of the mRNA vaccines Pfizer-BioNTech and Moderna and AstraZeneca based on studies that show higher vaccine efficacy for preventing severe disease hospitalization and death compared to all symptomatic disease. 95 cases in placebo group. Group sample sizes of 7545 SARS-CoV-2 vaccine and 3773 placebo suits at a two-sided 95 confidence interval for the difference in population proportions with a width equal to 10 when the estimated incidence rate for vaccinated group is 10 and the estimated incidence rate for placebo group is 20.
The COVID-19 vaccine Covaxin has shown an efficacy rate of 78 which is pretty good considering the circumstances and is 8 more than Serum Institute of Indias Covishield the other vaccine being available in the country. Sinopharm a business unit of China National Pharmaceutical Group initiated a Phase III study in the United Arab. Serbia looks east to fill coronavirus vaccine shortage So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. Phase 3 trials of Sinopharms vaccine showed an efficacy rate of 79 per cent against symptomatic Covid infection if the shots were administered three weeks apart. Vaccine efficacy against laboratory-confirmed symptomatic COVID-19 was estimated to be 78 in adults 18-59 years of age.
Actual Study Start Date. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose.
Adjuvanted inactivated vaccine produced in Vero cells and developed by India showed 81 efficacy in preventing COVID-19 after two doses. Nepal government had recently procured 800000 doses of Vero cell from Sinopharma under a government-to-government agreement with China the first of the consignments. Sinopharm Vero Cell Inactivated Covid 19 Vaccine.
A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. If anything the vaccine choice has added to the complication.
Sinopharms vaccine has an efficacy of 79 against symptomatic Covid infections the WHO says but its effectiveness among certain groups. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year. COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice.
COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. A b Liu R 3 February 2021.

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
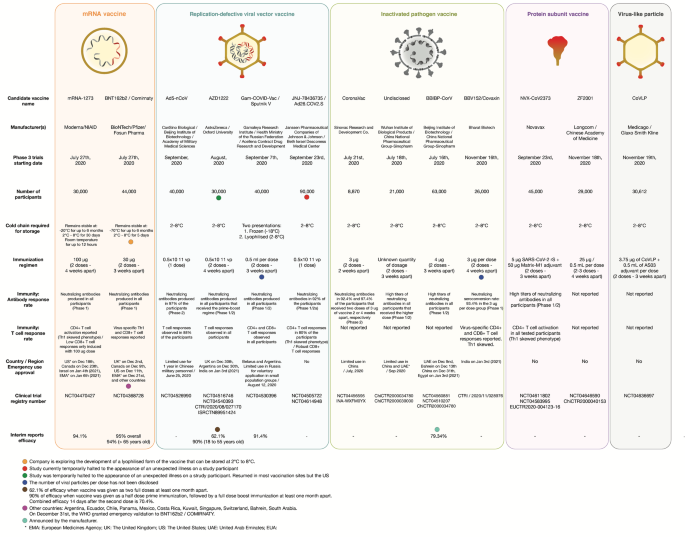
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021

China Grants First Approval Of Homegrown Covid 19 Vaccine Voice Of America English
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
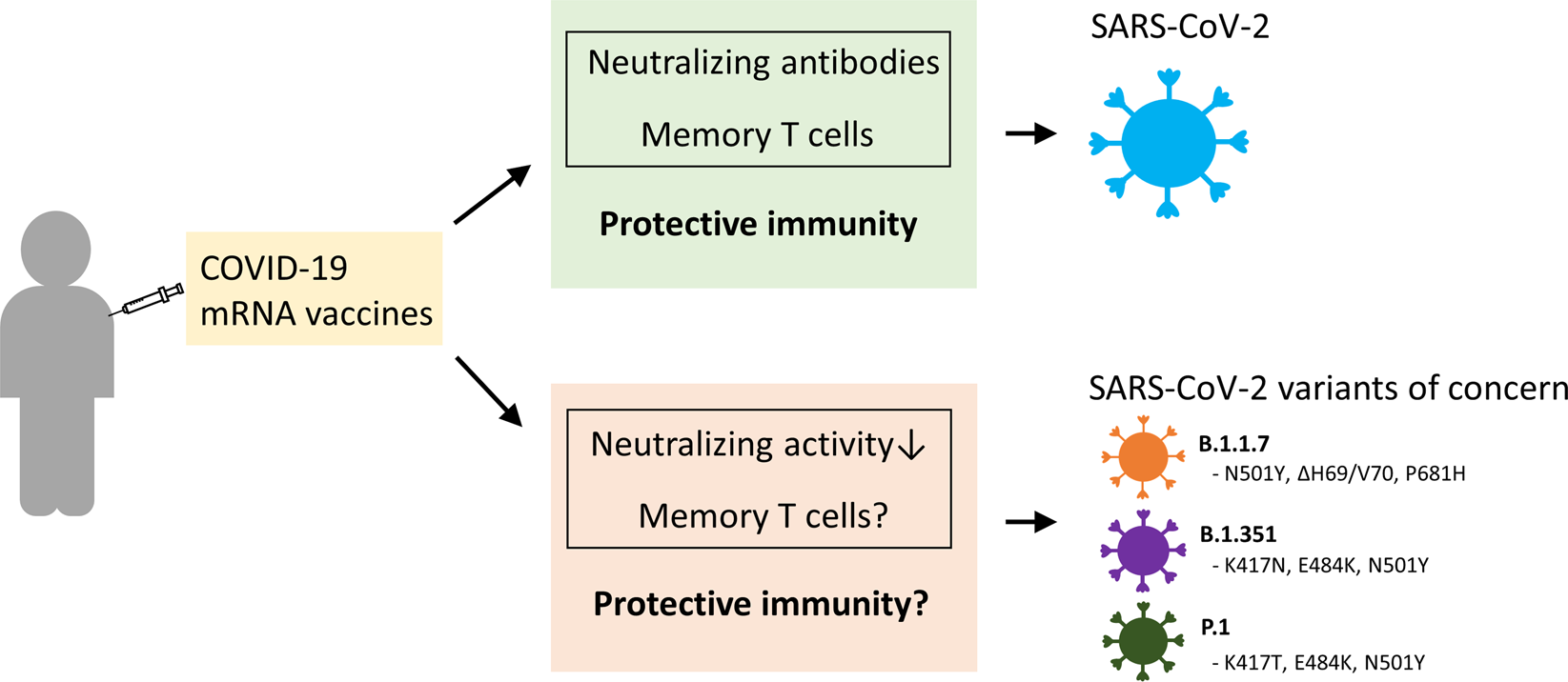
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use

Access To Covid 19 Vaccines Global Approaches In A Global Crisis