Vero Cell Covid Vaccine Efficacy Percentage / Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia
Vero cell vaccine efficacy against delta variant. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year.

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
The vaccine also induced T cell and memory.

Vero cell covid vaccine efficacy percentage. New analysis by PHE shows for the first time that 2 doses of COVID-19 vaccines are highly effective against hospitalisation from the Delta B16172 variant. 8849 Percentage of population fully vaccinated. Serbia looks east to fill coronavirus vaccine shortage So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac. EMA will assess the compliance of COVID-19 Vaccine Vero Cell Inactivated with the usual EU standards for effectiveness safety and quality.
Vaccine efficacy for symptomatic and hospitalized disease was estimated to be 79 all age groups combined. It also found it to be 100 effective in preventing severe disease as defined by the CDC and 953 effective in preventing severe disease as defined by the FDA. Percentage of population who received one dose.
The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. Cells used for vaccine manufacture are the working Vero cell bank which is of the 142nd generation. Results of a prospective randomized trial Vaccine.
Chinas Covid-19 Vaccine Vero Cell To Be Administered To 500000 People In Nepal Nepal has granted emergency approval to the Chinese Vero Cell Covid-19 vaccine due to the rising active. Few adults over 60 years were enrolled in clinical trials so efficacy could not be estimated in this age group. Vaccine efficacy against laboratory-confirmed symptomatic COVID-19 was estimated to be 78 in adults 18-59 years of age.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The PfizerBioNTech and OxfordAstraZeneca COVID-19 vaccines are effective against the highly infectious Delta variant of SARS-CoV-2 but their protection drops away over time a. On the basis of all available evidence WHO recommends the vaccine for adults 18 years and older in a two-dose schedule with a spacing of three to four weeks.
While EMA cannot predict the overall timelines it should take less time than normal to evaluate an eventual application because of the work done during the rolling review. A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Adjuvanted inactivated vaccine produced in Vero cells and developed by India showed 81 efficacy in preventing COVID-19 after two doses. Authors W Jaiiaroensup. Sinopharm vaccine efficacy for symptomatic and hospitalised disease was estimated to be 79 percent all age groups combined the who said.
In April the company announced the vaccine had 913 efficacy against COVID-19 based on measuring how well it prevented symptomatic COVID-19 infection seven days through up to six months after the second dose. Two efficacy trials are underway. Sinopharm a business unit of China National Pharmaceutical Group initiated a Phase III study in the United Arab.
JJ presented its interim results from a study of 20 people that shows that its vaccine. Safety and efficacy of purified Vero cell rabies vaccine given intramuscularly and intradermally. On December 29 2020 Sinopharm reported 79.
COVID-19 Vaccine Vero cell. The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. Vaccine efficacy for symptomatic and hospitalised disease was estimated to be 79 percent all age groups combined.
COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. The median duration of follow up available. 9th April 2021 1422 IST Generous assistance.
-19 Vaccine Covovax Covovax Vaccine Janssen Johnson Johnson Moderna mRNA-1273 Novavax Novavax Vaccine NVX-CoV2373. Bảo quản vắc xin. Referring to the above principles the SARS-CoV-2 inactivated vaccine Vero cells was administered once each on days 0 14 28 and 42 for a total of four administrations and the interval was 2 weeks followed by a recovery period after the last administration of 2 weeks 14 days.
While the efficacy rate of the AstraZeneca vaccine is at 66 percent. Vero cell vaccine efficacy against delta variant.

Access To Covid 19 Vaccines Global Approaches In A Global Crisis
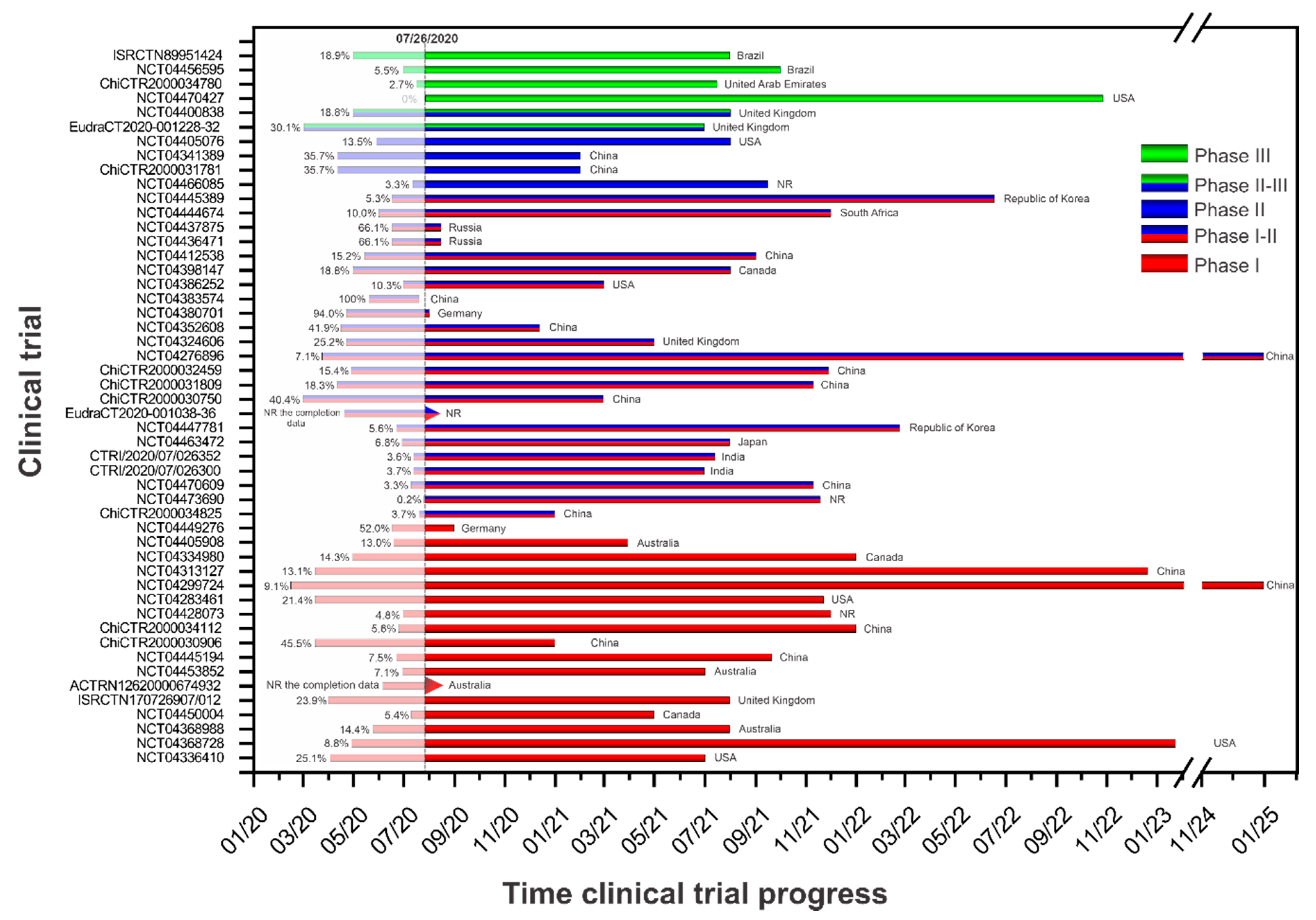
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
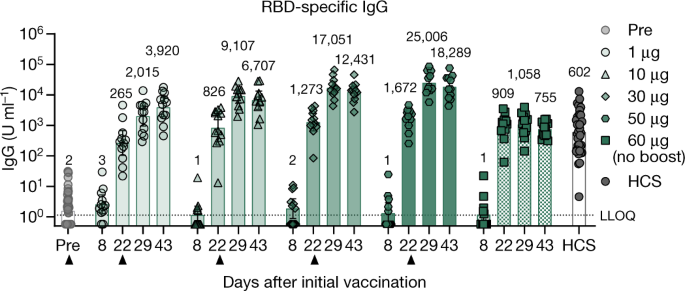
Covid 19 Vaccine Bnt162b1 Elicits Human Antibody And Th1 T Cell Responses Nature
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Efficacy And Safety Of An Inactivated Whole Virion Sars Cov 2 Vaccine Coronavac Interim Results Of A Double Blind Randomised Placebo Controlled Phase 3 Trial In Turkey The Lancet

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
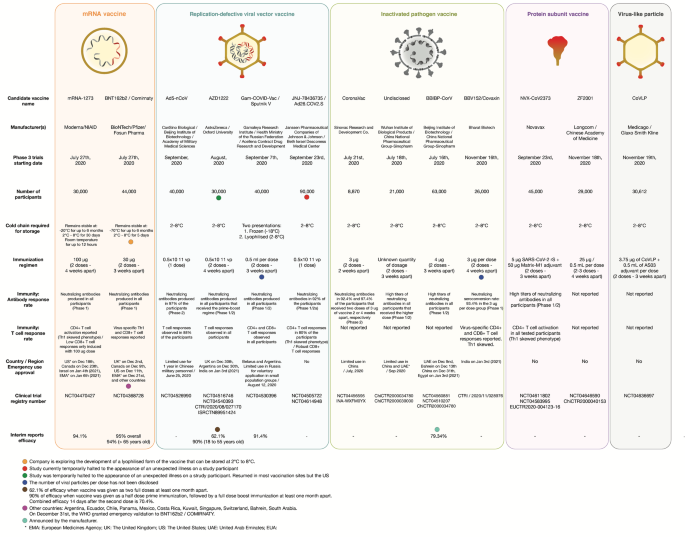
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

