Vero Cell Covid Vaccine Effectiveness / Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
The study is registered with ClinicalTrialsgov number NCT00566345. The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning.

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Analysis was by intention to treat.

Vero cell covid vaccine effectiveness. COVID-19 vaccine efficacy from clinical trials COVID-19 vaccines licensed for use in the EUEEA have been shown during clinical trials to be highly effective in providing protection against symptomatic COVID-19 and severe disease. When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. To evaluate the efficacy of whole course immunization for COVID-19 patients with clinical symptoms and confirmed by RT-PCR after two weeks.
To evaluate the safety of the vaccine within 7 days after each dose. The world has changed dramatically from the impact of the COVID-19. Vaccine efficacy is typically proved by large clinical trials over several years.
WHO Package Insert vaccine vials WHO Package Insert pre-filled syringes. That compared with 93 effectiveness against the Alpha variant first identified in Britain. It has impacted the normality of daily life highlighting the failure of rich and poor nations alike which is evident from the high number of human lives lost in rich and powerful countries like the USA with total deaths of 32735704 and Europe with 43708958 until April 24 2021 as per Worldometer.
Primary objective to evaluate the protective efficacy of inactivated sars cov 2 vaccine vero cell after full course of immunization in preventing diseases caused by the sars cov 2 in healthy subjects aged. Its easy storage requirements make it highly suitable for low-resource settings. COVID-19 Vaccine Vero Cell Inactivated Sinopharm CO1 accne Explaner 4 MAY 1 Recommended for age 18 years of age and above Vaccination of children or adolescents below the age of 18 years is not routinely recommended although the studies are underway.
Primary efficacy endpoint and analysis. The vaccine is authorized for use under the Prevention and Control of Disease Use of Vaccines Regulation Cap. Its effectiveness against COVID-19 is confirmed at 914 per cent while its efficacy against severe cases of the disease is 100 per cent.
What is CoronaVac and what it is used for1. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru - Full Text View. The vaccine was registered under the emergency use authorisation based on Russias Phase III clinical trials on more than 33000 participants.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. The COVID-19 Vaccine Vero Cell Inactivated is made from the SARS-CoV-2 19nCoV-CDC-Tan-HB02 strain which is inoculated on the Vero cells for culturing harvesting β-propiolactone-inactivation concentration and purification then followed by adsorption with aluminium hydroxide adjuvant to form the liquid vaccine. While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people who received two full doses and closer to 90 percent in.
Table 1 shows the overall vaccine efficacy of the licensed vaccines after completed vaccination. Ugm Expert Clinical Trials The Way To Prove Covid 19 Vaccines Effectiveness In Indonesia Universitas Gadjah Mada from ugmacid Maybe you would like to learn more. The vaccine is free of antibiotics and preservatives.
Real-world test-negative analysis in Bahrain based on 14 days post 2nd dose indicated a vaccine effectiveness of 90 95 CI 88 91 for adults aged 1859. CoronaVac is indicated for active immunization against COVID-19 disease caused by SARS-CoV-2 virus. Recommended schedule 2 doses 05 mL each at a recommended interval of 3 to 4 weeks.
Factsheet for Vaccination of CoronaVac COVID -19 Vaccine Vero Cell Inactivated. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. In the case of the Sinopharm vaccine the WHO assessment included on-site inspections of the production facility.
The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. The vaccine is based on human adenoviral vector-based platform. The primary objective was the efficacy of the vaccine in preventing cell-culture-confirmed influenza infection with viruses that were antigenically matched to one of the vaccine strains.
COVID-19 Vaccine Vero Cell Inactivated. Recommendation for an Emergency Use Listing EUL of SINOVAC Covid-19 vaccine Vero cell Inactivated - CoronaVac TM Published. Two doses of the AstraZeneca AZNL vaccine were 60.
COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. Vero Cell Vaccine Covid-19 Efficiency.

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
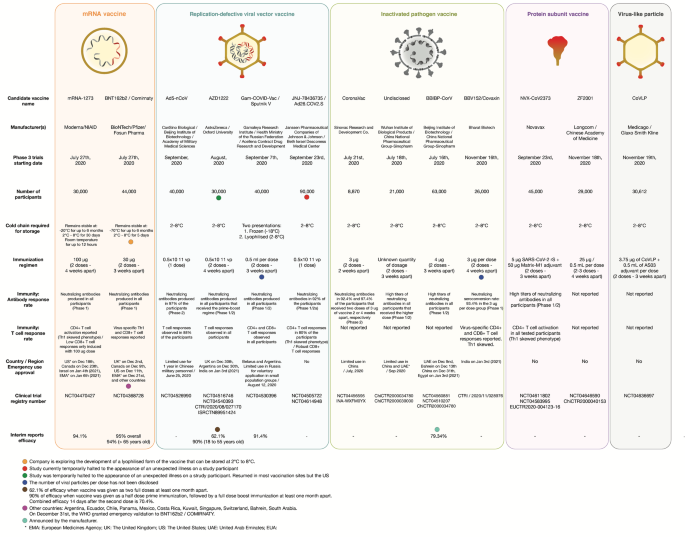
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
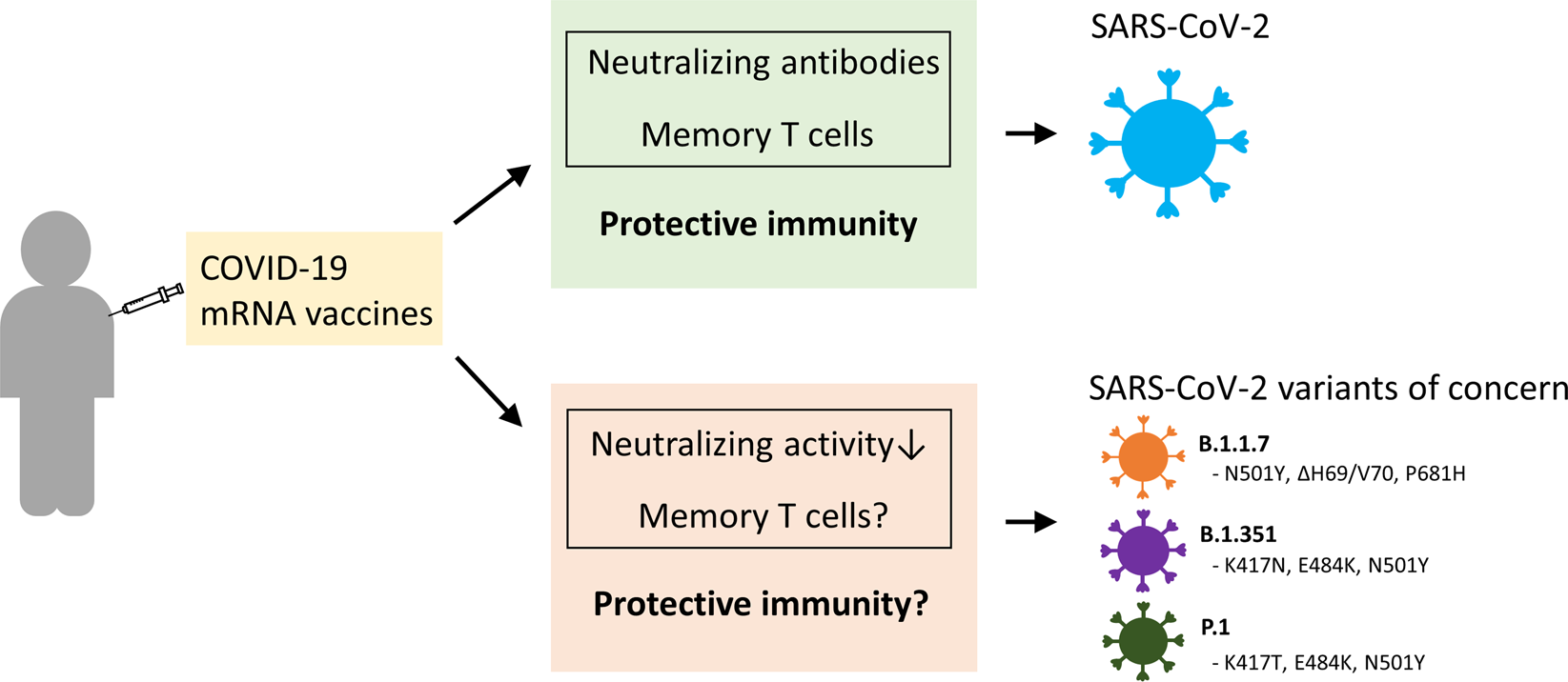
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
